Staff
Prof. Dr. Daigo Sumi
Dr. Hitomi Fuzishiro
Research projects
The name of our laboratory is “Molecular Nutrition and Toxicology”. We are focusing on molecular nutrition and toxicology of metals. Metals such as zinc (Zn), iron (Fe), and copper (Cu) are known to be involved in more than 10% of gene products as enzymatic co-factors or structural components. Dietary deficiency of a certain metal as a trace element causes nutritional deficiency syndromes, whereas excess intake of the same metal causes toxic health effects. To uncover whole profiles of biological actions and roles of metals, which are related to both nutrition and toxicity, application of multiple tools in the fields of genetic, biochemical, chemical, and epidemiological studies are required. In addition, we Japanese experienced heavy metal intoxication such as Minamata-disease and Itai-itai disease, but even 50 years after the discovery of these diseases, molecular mechanisms of metal toxicity and transport remain unclear.
The major projects carried out in our laboratory are as follows.
1. Clarification of molecular mechanisms of metal transport and its roles in metal toxicity:
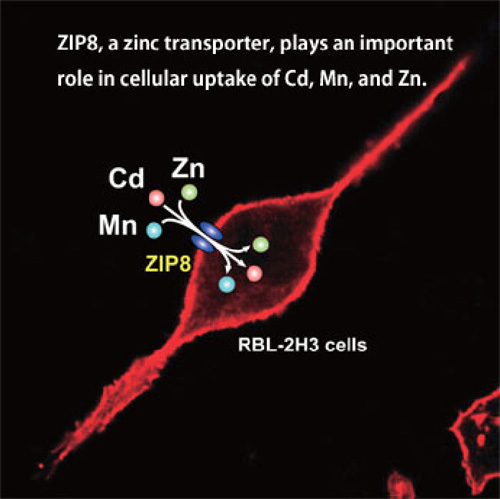
By developing Cd- and Mn-tolerant cell lines, we identified several zinc transporters such as ZIP8 and ZIP14 involved in the transport of Cd and Mn. (Ref. 4, 5, 9, 10) (See Fig. 1) We are currently studying the expression and roles of these metal transporters in the kidney, the target organ of Cd, and nervous system, the target organ of Mn. The interactions of Cd and other metals such as Mn and Co are also being studied.(Ref. 4, 5, 8 )
2. Clarification of molecular mechanisms of arsenic toxicity and metabolism — from molecular to clinical and epidemiological studies:
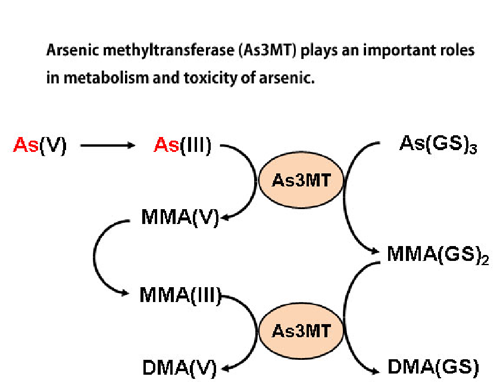
Arsenic is a well-known toxic metal, but the molecular mechanisms of its toxicity and metabolism remain still unclear. Some arsenic compounds are used as a pharmaceutical. We are currently studying the molecular mechanisms of arsenic toxicity and metabolism by using cultured cells and human samples obtained in arsenic-polluted areas in Bangladesh and Cambodia. (Ref. 1, 2, 3, 6, 7) (See Figs 2 and 3) Clarification of molecular mechanisms of biological actions of arsenic may also pave the way to apply arsenicals to clinical use in more efficient and safer way.
Publications
- Ogawa ,M., Okamoto, Y., Himeno, S., Suzukawa,K., Sumi, D. Arsenite suppresses the transcriptional activity of EVI1 through the binding to CCHC-type Zn finger domain. Biochem Biophys Res Commun. 2020 Sep 3;529(4):910-915. doi: 10.1016/j.bbrc.2020.07.024. Epub 2020 Jul 29.
- Sumi,D., Tsuyama, H., Ogawa,T., Ogawa, M., Himeno, S. Arsenitesuppresses IL-2-dependent tumoricidal activities of natural killer cells. Toxicol Appl Pharmacol. 2021 Feb 1;412:115353. doi: 10.1016/j.taap.2020.115353. Epub 2020 Dec 8.
- Rahman, A., Islam, M. S.,Tony, S. R., Siddique, A. E., Mondal, V., Hosen, Z., Islam, Z., Hossain, M. I., Rahman,M.,Anjum,A., Paul, S. K., Hossen, F.,Sarker, M. K., Hossain, S., Salam, K. A., Haque, A.,Hoque, M. A.,Saud, Z. A., Xin, L.,Sumi,D., Himeno,S., Hossain, K. T helper 2-driven immune dysfunction in chronic arsenic-exposed individuals and its link to the featuresof allergic asthma. Toxicol Appl Pharmacol. 2021 Apr 9;115532. doi: 10.1016/j.taap.2021.115532.
- Fujishiro, H., Taguchi, H., Hamamo, S., Sumi,D., Himeno, S. 41. Comparisons of segment-specific toxicity of platinum-based agents and cadmium using S1, S2, and S3 cells derived from mouse kidney proximal tubules. Toxicology in vitro, 75: 105179. 2021 doi:10.1016/j.tiv.2021.105179. PMID: 33905841.
- Sarker, M. K., Tony, S. R., Siddique, A. E., Haque, N., Islam, M. S., Hossain, F., Islam, Z., Hossain, S., Hoque, M. A., Saud, Z. A.,Sumi, D., Himeno, S., Hossain, K. Gender differences in the risk of metabolic syndrome among chronic arsenic-exposed individuals in Bangladesh. Expo Health. (in press) 2021.
- Fujishiro, H., Yamamoto, H., Otera, N., Oka, N., Jinno, M., Himeno, S. In vitro evaluation of the effects of cadmium on endocytic uptakes of proteins into cultured proximal tubule epithelial cells. Toxics 8(2), E24 (2020) PMID: 32244724 PMCID: PMC7356949 DOI: 10.3390/toxics8020024
- Sumi, D., Yoshino, Y., Kameda, R., Himeno, S. Chronic exposure to submicromolar arsenite promotes the migration of human esophageal Het1A cells induced by heparin-binding EGF-like growth factor. Arch. Toxicol. 93(12), 3523 – 3534. (2019) [PubMed]
- Fujishiro, H., Hamao, S., Isawa, M., Himeno, S. Segment-specific and direction-dependent transport of cadmium and manganese in immortalized S1, S2, and S3 cells derived from mouse kidney proximal tubules. J. Toxicol. Sci. 44(9), 611-619. (2019) [PubMed]
- Fujishiro, H., Himeno, S. Gene expression profiles of immortalized S1, S2, and S3 cells derived from each segment of mouse kidney proximal tubules. Fund. Toxicol. Sci. 6(4), 117-123. (2019) [J-STAGE]
- Tsuyama, H.,Fujishiro, H., Himeno, S.,Sumi, D.Arsenite suppresses NO production evoked by lipopolysaccharide and poly(I:C) via the suppression of interferon-β expression in RAW264.7 cells. J. Toxicol. Sci. 44(2), 83-92. (2019) [PubMed]