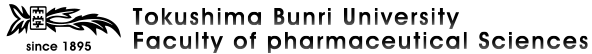Staff
Prof. Dr. Hiroto Kaku
Dr. Kei Kitamura
Research project
Publications
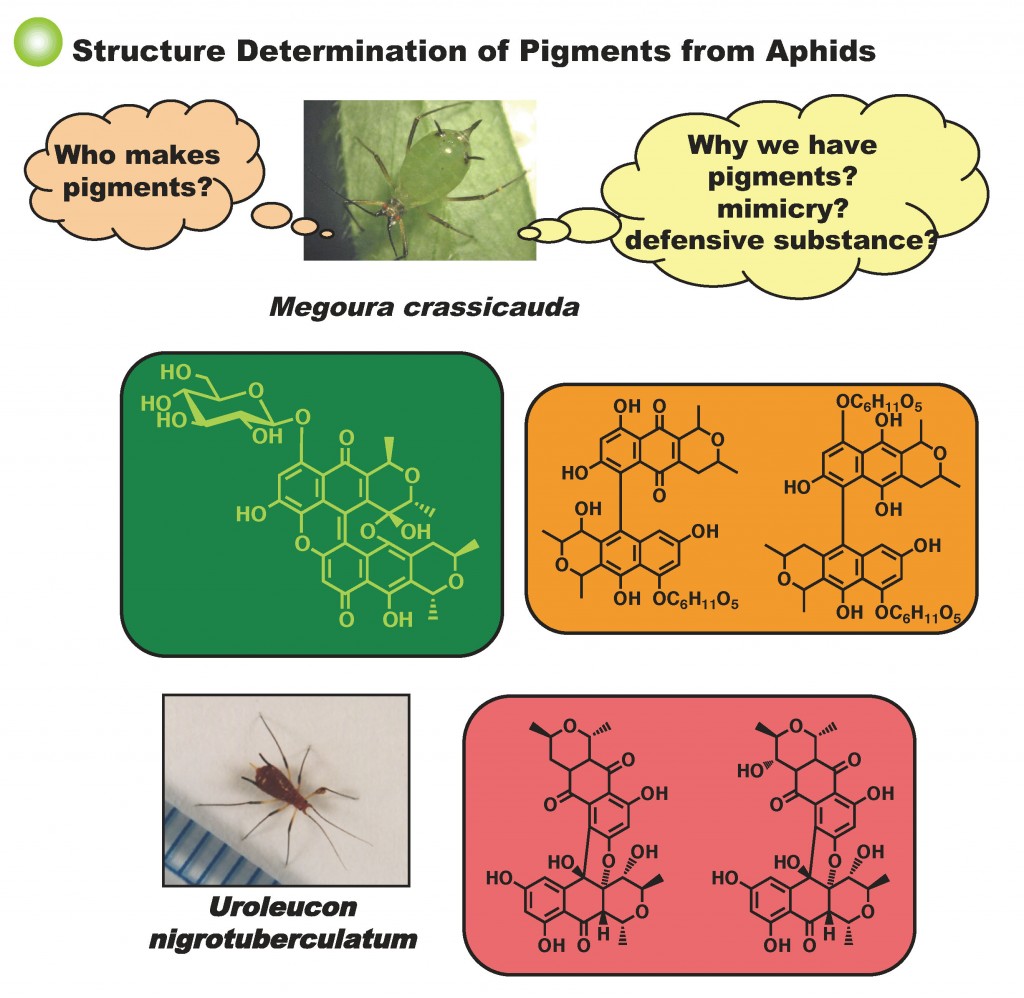
- Strong acid-promoted skeletal remodeling of the aphid pigment: red uroleuconaphin to green viridaphin, Chiharu Ozakai, Kei Kitamura, Mitsuyo Horikawa, To-sho Hoshiyama, Akari Imamura, Tatsuro Yoneyama, Akemi Umeyama, Masaaki Noji, Tetsuto Tsunoda, Hiroto Kaku, New J. Chem., 2022, 46, 2600-2604. DOI:10.1039/D1NJ05261F
- Synthesis of the Common Monomeric Unit of Uroleuconaphins and Viridaphins via Hauser–Kraus Annulation,
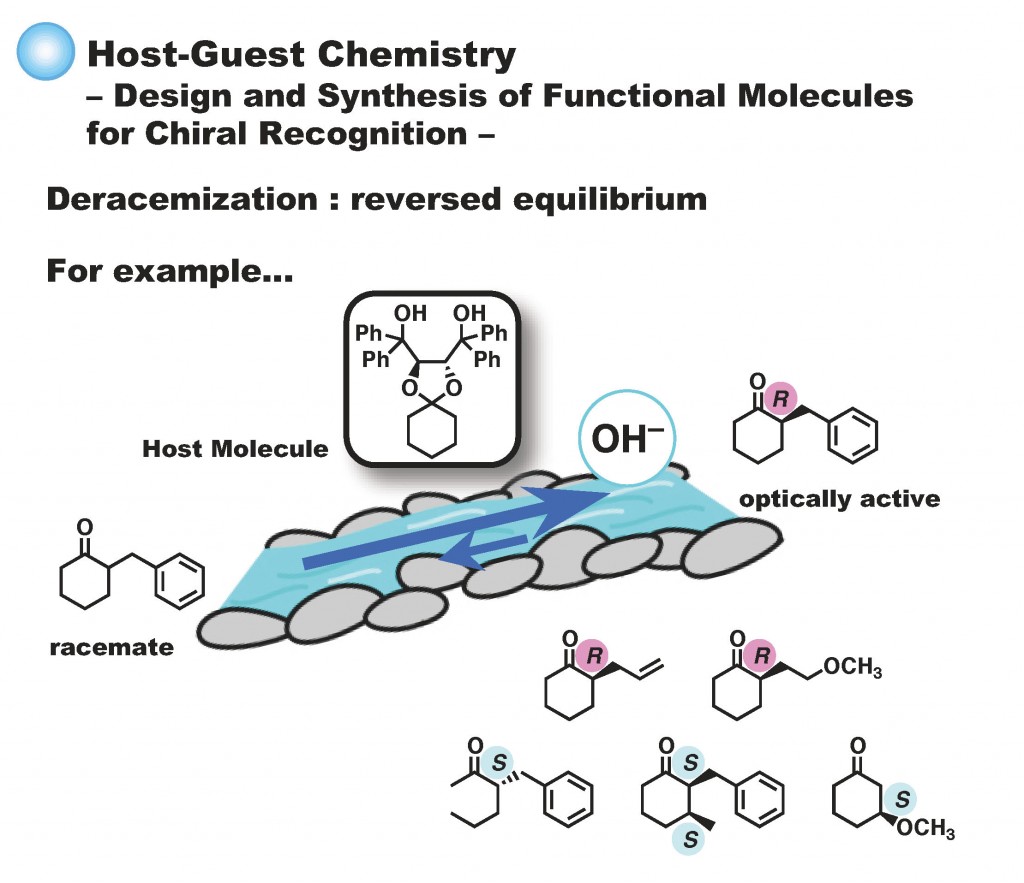
Kei Kitamura, Hinano Kanagawa, Chiharu Ozakai, Taichi Nishimura, Hayato Tokuda, Tetsuto Tsunoda, Hiroto Kaku, SYNTHESIS, 2021, 53 (9), 1629-1635. DOI:10.1055/a-1334-6982 - Total Syntheses and Cytotoxic Evaluations of Cryptolactones A1, A2, B1, B2, and Their Derivatives, Makoto Inai, Yoki Oguri, Mitsuyo Horikawa, Hiroto Kaku, Shinya Suzuki, Kei Kitamura, Tetsuto Tsunoda, Chem. Pharm. Bull., 2020, 68 (4), 380-383. DOI:10.1248/cpb.c19-01114
- Piperidine and Azetidine Formation by Direct Cyclization of Diols with N-Nonsubstituted Sulfonamide under the Mitsunobu Conditions Utilizing (Cyanomethylene)tributylphosphorane (CMBP) and Its Application to the Synthesis of Lupinine, Hiroto Kaku, Yuhei Sonoda, Hideyuki Hishida, Yuri Taniguchi, Akiko Kubo, Takumi Hamaguchi, Mitsuyo Horikawa, Makoto Inai, Kei Kitamura, Tetsuto Tsunoda, HETEROCYCLES, 2019, 98 (11), 1525-1535. DOI:10.3987/COM-19-14171
- Optically Active 2,7,10,15-Tetrahydroxytetraphenylene: Clathrates with Both Enantiomers of 1-Phenylethylamine and Their Stability, Hiroto Kaku, Akinobu Mitarai, Natsuko Okamoto, Kenta Tanaka, Sayaka Ichikawa, Takahiro Yamamoto, Makoto Inai, Takeshi Nishii, Mitsuyo Horikawa, Tetsuto Tsunoda, Eur. J. Org. Chem., 2018, 48, 6991-6999. DOI:ejoc.201801422
- A role of uroleuconaphins, polyketide red pigments in aphid, as a chemopreventor in the host defense system against infection with entomopathogenic fungi, Mitsuyo Horikawa, Mitsuaki Shimazu, Maki Aibe, Hiroto Kaku, Makoto Inai, Tetsuto Tsunoda, J. Antibiotics, 2018, 71, 992–999. DOI:10.1038/s41429-018-0093-4
- 3,3-Dimethoxypropylsulfonyl Group: A new versatile protecting and activating group for amine synthesis, Izumi Sakamoto, Kazuya Iwaoka, Yuta Kawada, Takanori Naito, Kazuyoshi Makida, Yuki Takeuchi, Takeshi Nishii, Mitsuyo Horikawa, Hiroto Kaku, Tetsuto Tsunoda, Tetrahedron, 2018, 74 (24), 3052-3060. DOI:10.1016/j.tet.2018.04.088
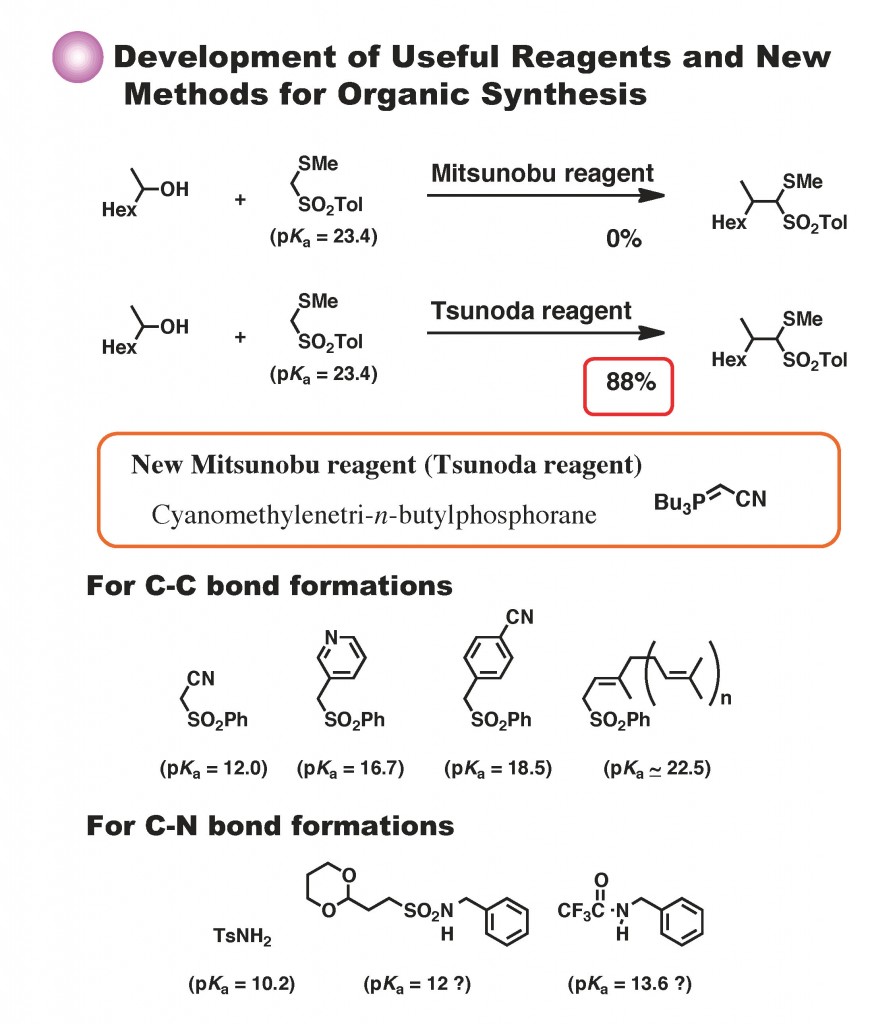
- Deracemization of α-monosubstituted cyclopentanones in the presence of a TADDOL-type host molecule, >Hiroto Kaku, Minami Ito, Mitsuyo Horikawa, Tetsuto Tsunoda, Tetrahedron, 2018, 74 (4), 124-129. DOI:10.1016/j.tet.2017.11.045